Acids, Bases, and pH
Learning Objectives
In this activity, students will become familiar with one of the most important chemical classification systems, pH, and they will use that knowledge to determine approximate pH values of some common household materials.
Materials
To prepare the cabbage indicator:
- Red cabbage
- Water
- Source of heat
- Strainer
For the activity:
- 7 small cups of cabbage solution
- Some chalk or antacids
- Baking soda
- Vinegar
- Lemon juice
- Water
- Dish detergent (something like Mr Clean that contains sodium hydroxide)
- Bleach (or Tilex, something that contains bleach)
- Acids and Bases worksheet (provided)
Motivation
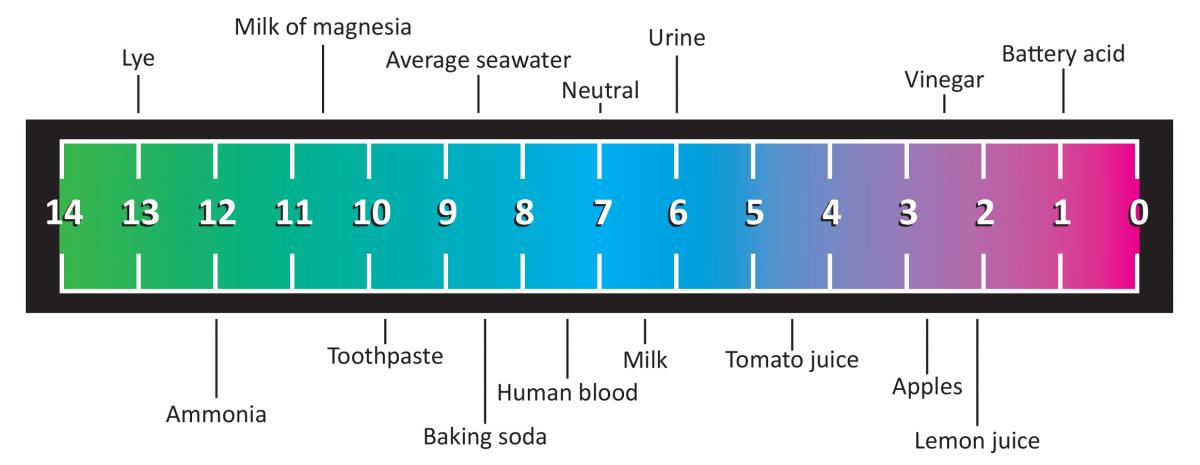
The measure of pH is a way to classify any chemical, based on its properties when it dissolves in water. All substances can be classified as neutral (with a pH of about 7), basic (pH greater than 7), or acidic (pH less than 7), and the pH tells us how strong or weak that substance is as well. For example a substance with pH = 8 is a very weak base, but a substance with pH = 3 is a strong acid. The closer you are to neutral, the weaker your substance is, acidic or basic. A common way to determine the pH of a substance is to use special chemicals called pH indicators. These are substances which change colour at different pH levels, and different indicators have different ranges of pH in which they are reactive.
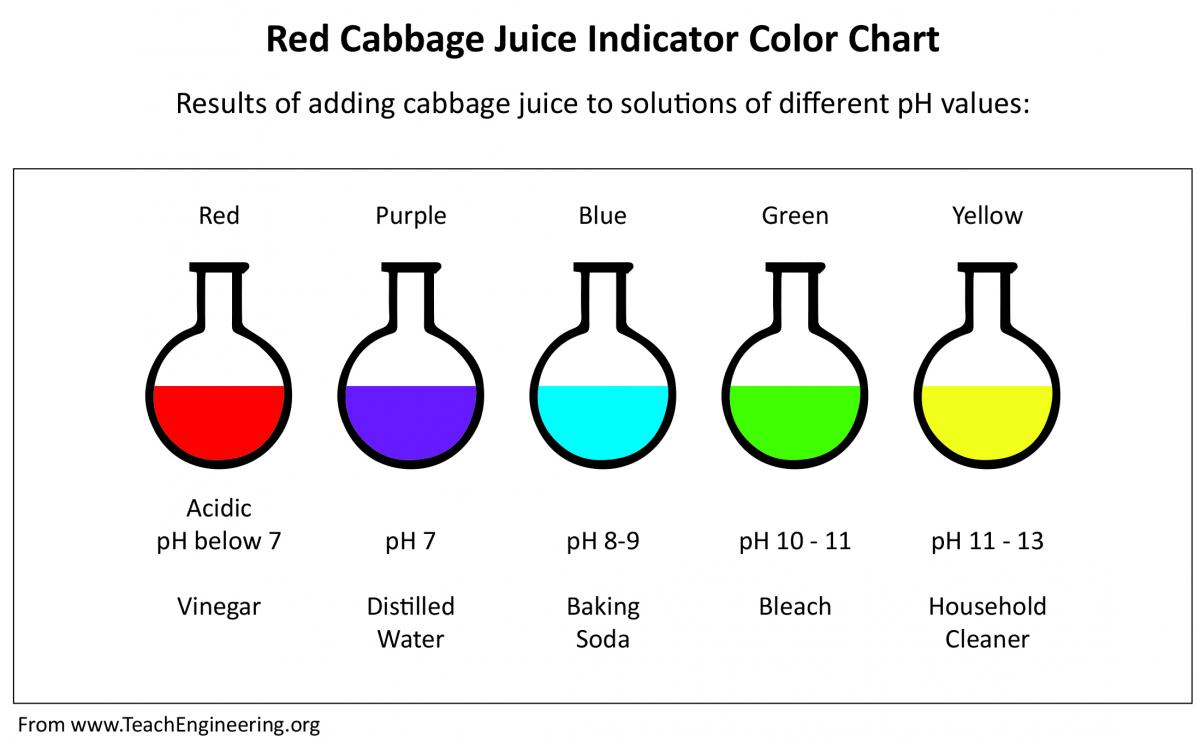
The cabbage juice solution is a very useful indicator, because it has a very wide range of pH levels to which it is reactive. What your students should find is that adding baking soda or antacids to the mixture will turn it purple. This is because baking soda and antacids are weak bases. Adding lemon juice or vinegar should give a bright red colour, because these are both acidic. Adding water should change the colour very little, if at all, because water is neutral. However, other chemicals in the water (like chlorine, or fluorine) can make the water more acidic or basic. If you have access to distilled water, it is recommended that you use this to make the cabbage juice. Dish detergent will turn the cabbage juice green, because it is a stronger base than baking soda or the antacids. Bleach will turn the cabbage juice bright yellow, because it is a very
strong base.
Procedure
Part 1
This part is best done outside of class, to make for a shorter set up time. First, you need to slice up the cabbage and boil the bits in about 2-3 cups of water. Boil until the juice is red-purple. At this point, the pH of the mixture is about 7. Then strain the vegetable chunks out of your juice.
Now, your pH indicator is ready for use.
Part 2
Set out 7 small cups of the juice for the groups to work with and provide them with the necessary worksheet. Add one of each substance to each cup and make note of the change in colour. The following make good substances to use: lemon juice, vinegar, chalk, baking soda,
water, dish detergent, and bleach.
Part 3
Make note of the changes in colour and, using the provided chart, make an estimate of the pH of each substance.
Careful
The pH readings on this experiment will NOT be perfect. This is because the pH of your cabbage juice solution will depend on how much water you use, and the initial pH of that water. However, the colour changes will be exciting, and this is still an excellent way to introduce
students to elementary chemistry.
Investigating Questions
- How can we determine the pH of a substance?
- What is a pH indicator?
- What pH might a strong base have?
- What pH might a weak acid have?
pH Scale